University of Michigan develops efficient system for converting CO2 into ethylene
by Clarence Oxford
Los Angeles CA (SPX) Sep 19, 2024
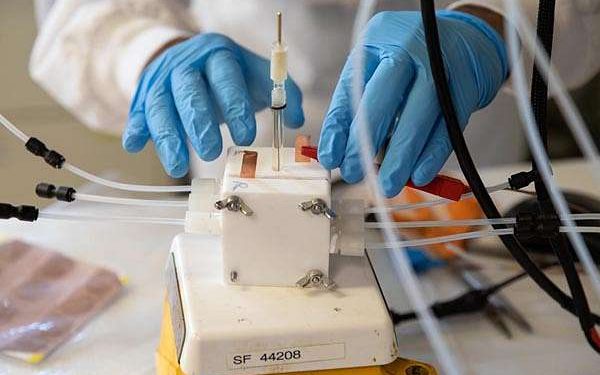
Researchers at the University of Michigan have made a significant advancement toward creating sustainable fuels by developing an artificial photosynthesis system that efficiently chains carbon atoms together. The system is capable of converting carbon dioxide into ethylene, a critical hydrocarbon used in plastics, with field-leading efficiency, yield, and longevity.
“The performance, or the activity and stability, is about five to six times better than what is typically reported for solar energy or light-driven carbon dioxide reduction to ethylene,” said Zetian Mi, a professor of electrical and computer engineering at the University of Michigan and the corresponding author of the study, which was published in ‘Nature Synthesis’.
Ethylene, the most widely produced organic compound in the world, is traditionally created using oil and gas under high temperatures and pressures – processes that contribute significantly to carbon dioxide emissions. By utilizing this new photosynthesis system, it may become possible to produce ethylene without adding to atmospheric CO2 levels.
The long-term goal of the research team is to develop a process that chains more carbon and hydrogen atoms together, potentially leading to the creation of liquid fuels, which are easier to transport and could support sustainable energy solutions.
The device created by the Michigan team uses two types of semiconductors: a base layer of silicon with gallium nitride nanowires grown on top. These nanowires, each just 50 nanometers wide, are dotted with copper clusters that catalyze the conversion of water and carbon dioxide into ethylene.
When exposed to light, the semiconductors generate electrons that break apart water molecules, producing hydrogen for the reaction. The copper clusters then facilitate the bonding of carbon atoms from carbon dioxide into carbon monoxide, eventually leading to the creation of ethylene.
The device stands out not only for its efficiency but also for its durability. While previous systems lasted only a few hours, the Michigan team’s device ran continuously for 116 hours without losing performance. Some earlier iterations have operated for up to 3,000 hours. This longevity is attributed to the synergistic effects between gallium nitride and the water-splitting process, which leads to self-healing of the catalyst over time.
Looking ahead, the research team plans to explore ways to extend the process to create other multicarbon compounds, including propanol, as they work toward the ultimate goal of producing sustainable liquid fuels.
“In the future, we want to produce some other multicarbon compounds such as propanol with three carbons or liquid products,” said Bingxing Zhang, assistant research scientist at U-M and first author of the paper.
Research Report:Interfacially coupled Cu-cluster/GaN photocathode for efficient CO2 to ethylene conversion
Related Links
University of Michigan
All About Solar Energy at SolarDaily.com